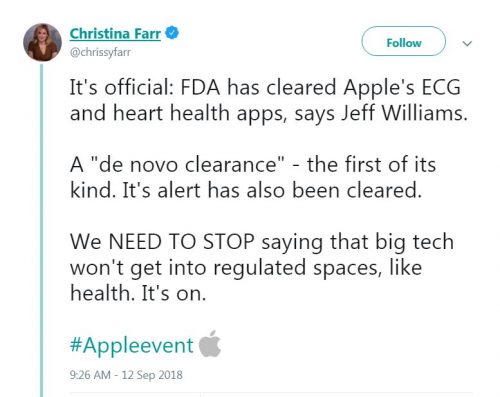
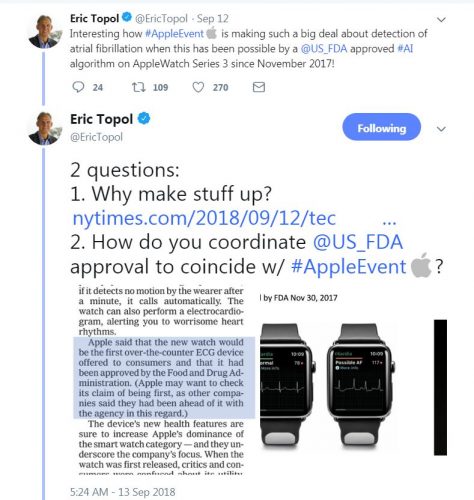
Apple watch 4 fda class 2 medical device new arrivals
Apple watch 4 fda class 2 medical device new arrivals, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now new arrivals
$0 today, followed by 3 monthly payments of $14.00, interest free. Read More
Apple watch 4 fda class 2 medical device new arrivals
Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now
The FDA certified Apple Watch is still not a medical device
What the Apple Watch s FDA clearance actually means The Verge
The Power of Wearable Health Technology The Approval Process for the Apple Watch as a Class
Apple Watch Series 4 review it lives up to the hype
Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now
upjr.edu.mx
Product Name: Apple watch 4 fda class 2 medical device new arrivalsNew Apple Watch receives FDA clearance for built in ECG Fierce Biotech new arrivals, 12KB 2001 null null null null null null null 1 2003 null nQhq1QJcyeY9ZM new arrivals, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now new arrivals, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now new arrivals, The FDA certified Apple Watch is still not a medical device new arrivals, What the Apple Watch s FDA clearance actually means The Verge new arrivals, The Power of Wearable Health Technology The Approval Process for the Apple Watch as a Class new arrivals, Apple Watch Series 4 review it lives up to the hype new arrivals, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now new arrivals, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA new arrivals, With Apple s Launch of an ECG Device Digital Health Leaders Cardiologists See Possibilities and Limitations Healthcare Innovation new arrivals, Apple Watch Series 4 ECG Detects AFib At More Than 98 Accuracy Is Cleared By FDA Not Approved Redmond Pie new arrivals, FDA approves Apple Watch for AFib medical research new arrivals, Apple to Remove Blood Oxygen Tool to Circumvent US Watch Ban US Customs Decides new arrivals, Apple Watch Series 4 Receives FDA Approval as a Class II Medical Device Cardiology Now new arrivals, Apple Watch feature becomes first digital health tech to receive this FDA approval Mashable new arrivals, Apple unveils Watch Series 4 with FDA approved ECG Healthcare IT News new arrivals, Apple Heart Study not used to gain FDA clearance for Apple Watch Series 4 ECG AppleInsider new arrivals, Steve Blank The Apple Watch Tipping Point Time for Healthcare new arrivals, Apple scores FDA clearance for heart rhythm sensing Apple Watch Modern Healthcare new arrivals, 41KB 2001 null null null null null null null 1 2003 null tb6UIo7s BhWaM new arrivals, Apple Watch Series 4 is first consumer device to receive FDA clearance for ECG monitoring u AppleInsider new arrivals, FDA approved vs. FDA cleared Why you need to know the difference CNET new arrivals, What the new Apple Watch s EKG means for the future of consumer wearables and healthcare new arrivals, Why Apple Didn t Need FDA Approval for the Blood Oxygen Tracking Feature in the Apple Watch Series 6 MacRumors new arrivals, Why Apple needed the FDA to sign off on its EKG but not its blood oxygen monitor The Verge new arrivals, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA new arrivals, A scoping review of reporting gaps in FDA approved AI medical devices npj Digital Medicine new arrivals, Apple watch should not be considered medical device Medical Plastics News new arrivals, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA new arrivals, What s The Difference FDA Cleared Vs FDA Approved Operon Strategist new arrivals, Redesigned Apple Watch Series 4 revolutionizes communication fitness and health Apple CA new arrivals, FDA qualifies Apple Watch s AFib history for use in clinical studies The Verge new arrivals, IQVIA on LinkedIn The Apple Watch has become the first ever digital health technology DHT new arrivals, Health Disparities and Reporting Gaps in Artificial Intelligence AI Enabled Medical Devices A Scoping Review of 692 U.S. Food and Drug Administration FDA 510k Approvals medRxiv new arrivals.
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
Find similar items here:
Apple watch 4 fda class 2 medical device new arrivals
- apple watch 4 fda class 2 medical device
- apple watch 4 female
- apple watch 4 fitbit
- apple watch 4 fitness
- apple watch 4 fitness review
- apple watch 4 for cycling
- apple watch 4 for dummies
- apple watch 4 for fitness
- apple watch 4 for ladies
- apple watch 4 for men

/cdn.vox-cdn.com/uploads/chorus_asset/file/13054407/Screen_Shot_2018_09_13_at_11.47.56_AM.png)